What Element Has 5 Electrons in the 3p Sublevel
Between which type of elements do ionic bonds occur and how do electrons act within the bond. Consider the elements neon bromine and phosphorus.
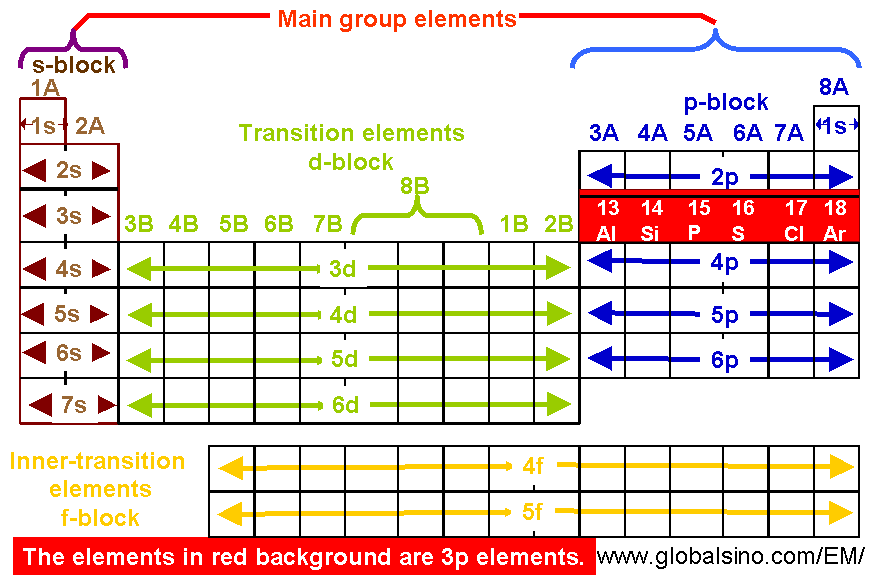
3p Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book
Phosphorus has three electrons in its 3p sublevel.
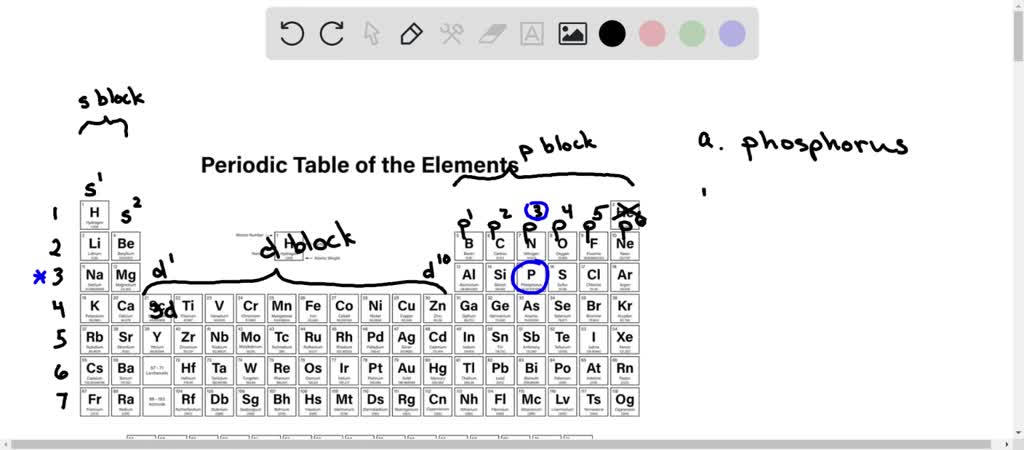
. Contains that first d. Express your answer as an integer. Aluminum also has them.
Part B has three 2p electrons Express your answer as a chemical symbol. The element that has the three half filled orbitals in 3P sublevel is. Thallium Tl The atomic number of this atom is 81 and number of electrons that are present are 81.
Give the symbol of the element that meets the following conditions. Part a-10 part b-1 part c-4. Helium atoms have 2 valence electrons while atoms of the other elements in the group all have 8 valence electrons.
The group 17VIIA elements have five electrons in their outermost highest energy p sublevel. This element has 1 electron in its 3d sublevel Sc. Which has the highest occupied energy level.
I think it should be clear now which element has two unpaired electrons in. Give the symbol of the element that meets the following conditions. 5 atomic orbitals in 5d sublevel x 2 electronsorbital 10 electrons can reside in the 3d sublevel Add up the number of electrons in step 6 to get a total of.
Phosphorus electronic configuration is 1S2 2S2 2P6 3S2 3P3 or Ne 3S2 3P3. The highest occupied energy level. According to your information there are five electrons in the 5p sublevel in which two of the three 5p orbitals contain paired electrons with the third 5p orbital containing one unpaired electron for a total of five represented as 5p5.
This element does not have 3 electrons in 4d-subshell. Identify the elements that have the following electron configurations. This element would be iodine.
The fourth shell has 4 subshells. This element does not have 3 electrons in 4d-subshell. The d sublevel has 5 orbitals so can contain 10 electrons max.
6Which element has a half-filled sublevel. How many 3p electrons are in S. Hence the correct answer is Option B.
Metal 2metal 3nonmetal 4Z 5Z 6X. We review their content and use your feedback to keep the quality high. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d3 c.
This element has 5 electrons in its 5p sublevel I. Consider the elements neon bromine and phosphorus. Has 5 electrons in the 3p sublevel.
Express your answer as an integer. And the 4 sublevel has 7 orbitals so can contain 14 electrons max. Ne3s 2 3p 5.
Part A has five electrons in the 3p sublevel Express your answer as a chemical symbol. The electronic configuration of thallium is. 2 Write the electron configurations for.
1F or Cl 2Mg or. Three electrons in its 4p sublevel. Give the symbol of the element that meets the following conditions.
You may have mistaken Si for sulfur Si is silicon. The s subshell which has 1 orbital with 2 electrons the p subshell which has 3 orbitals with 6 electrons the d subshell which has 5 orbitals with 10 electrons and the f subshell which has 7 orbitals with 14 electrons for a. Metals and metals electrons freely moving B.
Select the element in each pair with the higher ionization energy. Which has three electrons in is 3p sublevel. Which has its highest energy level completely filled.
View the full answer. Ne3s 2 3p 2 Cl. Configuration 1s2 2s2 2p6 3s2 3p3.
Part D How many 5s electrons are in Sr. 1s2 2s2 2p6 3s2 3p6 4s1 b. The s sublevel has just one orbital so can contain 2 electrons max.
Chlorines 3p sublevel has 5 electrons in it. The p sublevel has 3 orbitals so can contain 6 electrons max. The ____________ principle is the principle of quantum physics that states that it is impossible to determine accurately both the position and the speed of an electron at the same time.
Metals and nonmetals. Its highest energy level is completely filled. A Co 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7 b Y 2 Kr5s 0 4d 1 c V 4 Ar4s o 3d 1 d Se - Ar4s 2 3d 10 4p 5 e Fe Ar4s 1 3d 6.
Ne3s 2 3p 3 Mg. 5 electrons in the 3p su. Phosphorus P Phosphorus is in group 15 period 3 in the periodic table hence it is in atomic number 15.
Has 3 2p electrons. In the picture below the orbitals are represented by the boxes.

Solved A This Element Has A 3p Sublevel That Contains 3 Electrons B This Element Has A 4s Sublevel With 2 Electrons For Its Outermost Electrons C This Element Has 5 Electrons In

Solved A This Element Has A 3p Sublevel That Contains 3 Electrons B This Element Has A 4s Sublevel With 2 Electrons For Its Outermost Electrons C This Element Has 5 Electrons In

Table 02 Chemistry Lessons Chemistry Education Electron Configuration
Belum ada Komentar untuk "What Element Has 5 Electrons in the 3p Sublevel"
Posting Komentar